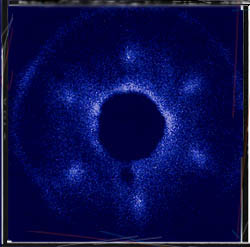
Okay, time for some review. If you had to guess, what state of matter is shown in this atomic force image? Hint: It is crystalline. You can tell because the bright spots (atoms) are in very ordered, symmetrical positions. Ask yourself: will this material hold its own shape or take on the shape of its container?
Links to addition information are in blue with underlining. These additional notes can be really helpful : )
| Week |
Topic |
Nevada Science Standards |
Activity |
Online Resources |
| 1 |
Nature of Science: How is Science different than other ways of knowing? |
N.12.A.1: Using Data N.12.A.2: Record Keeping N.12.A.3: Repeated Data N.12.A.5: Use of models |
LAB 1: |
Copy of Lab in PDF |
| 2 |
Types of matter Physical properties of matter.
Measuring and Lab Techniques.
|
N.12.A.1: Using Data N.12.A.2: Record Keeping N.12.A.3: Repeated Data N.12.B.1: Science & Society
|
LAB 2: |
Density Homework |
| 3 |
|
N.12.A.4: Safely conduct original lab investigation |
Density
Demo: Flag in a beaker. |
Scientific Notation Homework |
| 4 | States of matter
|
N.12.A.1: Charts and graphs are used in science P.12.A.1: Using Data P.12.A.3: Properties of mixtures can be used to separate or identify them
|
Quiz: Matter, Density, Scientific Methods LAB 3:Mix Mix Same Old Tricks
|
|
| 5 |
Compounds vs Mixtures Laws and Theories: What's the diff? Conservation of mass: it's a law |
P.12.A.3: Separation of mixtures P.12.A.7: Conservation of mass, small ratios of atoms |
LAB 4: You're in Hot Water Now
LAB 5: |
Chapter 3 Phase Changes Homework |
| 6 | Atomic Theory of chemistry History and Key discoveries and what they mean to us Complete this Early developments worksheet for 25 points extra credit. |
N.12.B.3: Science builds on past knowledge P.12.A.2: Elements in the periodic table are arranged into groups and periods by repeating patterns and relationships. P.12.A.9: The number of electrons (relative to protons) determines whether a given atom is neutral or charged (an ion) |
Flame Test Demos. |
Atomic Theory of Chemistry worksheet |
| 7 | Periodic
Table and Periodic Table assignment.
|
P.12.A.2: Elements in the periodic table are arranged into groups and periods by repeating patterns and relationships. | Periodic Table Assignment. | Elements crossword (print completed puzzle for 50 e.c. points) |
| 8 |
Review: Periodicity and patterns in the Periodic Table of Elements
|
P.12.A.3: Separation of mixtures P.12.A.7: Conservation of mass, small ratios of atoms P.12.A.2: Elements in the periodic table are arranged into groups and periods by repeating patterns and relationships. P.12.A.9: The number of electrons (relative to protons) determines whether a given atom is neutral or charged (an ion) |
Lecture Notes and Review Quiz Game.
Quiz: Compounds, mixtures, atomic theory, periodic table. |
|
| 9 |
Forming compounds:
Balancing charges
Chemical reactions: New
partners Bonding Types: Sharing or
stealing? |
P.12.A.4: Atoms
bond with one another by transferring or sharing electrons. E/S
P.12.A.5: Students know chemical reactions can take place at different rates, depending on a variety of factors (i.e. temperature, concentration, surface area, and agitation). E/S P.12.A.6: Students know chemical reactions either release or absorb energy. E/S |
Lab
6: All in the family: Periodic Trends.
Cornstarch demo/CuSO4 solution rates. |
Chapter 4 Homework: Covalent Bonding: Names and Formulas |
In addition to the topics listed, expect a quiz approximately every 2 to 3 weeks. It will not take the whole class period, and there may be opportunities for in-class review depending on student attitudes. The best way have success is to review labs, homework and reading before assigned tests. : )
For the second nine week's schedule click HERE
To report a dead or missing link click here: