Ok, what is it with this whole Chemistry thing?

Well I suppose the starting point is this: Chemists separate the vast world of matter (everything that takes up space and has mass into two giant categories: Pure Substances and Mixtures.
Pure substances
 (Polly Purebred)
(Polly Purebred)
Now pure doesn't mean moral, or nutritious or
good (like our Polly Purebred above). To a chemist pure means only
one thing. Made from only 1 kind of molecule.
Compounds
Take for instance sugar yum!!! : )
 We
can see that the sugar crystals are pure. Only the very best for you. But by
reacting sugar with
We
can see that the sugar crystals are pure. Only the very best for you. But by
reacting sugar with
other chemicals we know that sugar is made of molecules
(different atoms bonded together to make a new substance).
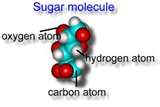
Every molecule of sugar has carbon and oxygen
and hydrogen in very specific ratios. If fact the simplest sugar has a
chemical formula of C6H12O6
. All of these atoms are bonded together and act as a unit. This is the
smallest unit that
has the properties of sugar. A pure substance made of
molecules with more than one type of atom is called a compound.
Elements
The second class of pure substances are
elements. Many of these don't really have molecules (just big piles of the same
type of atom). Those that have molecules are made of just
one type of atom. That is what makes them an element. They
cannot be broken down to anything simpler by normal
chemistry. What could be simpler than having just one type of part?
How about some stinky sulfur for example: 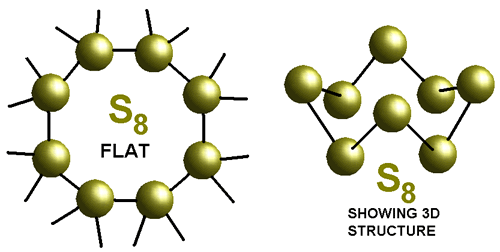 Its
an S8 molecule, but every atom is S. So its
Its
an S8 molecule, but every atom is S. So its
an element. Elementary right??? : )
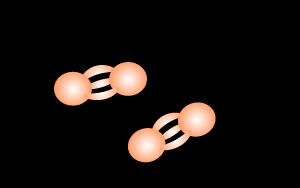 Or N2 molecules
which is 80% of our air. Again, another element, 'cause even though its a
Or N2 molecules
which is 80% of our air. Again, another element, 'cause even though its a
molecule it only has one type of atom.
Many metals, like zinc, shown below are just
piles of atoms packed as closely as possible. They are of no particular size and
aren't really considered molecules. 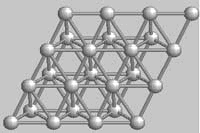 Zinc
crystal
Zinc
crystal
Mixtures
Mixtures aren't pure substances. They have at least 2 kinds of molecules. Mixtures come in two varieties as well.
Inhomogeneous mixture
These mixtures have visible parts or layers.
Keep in mind this depends on the scale of observation. To you and me, a drop
of blood is all the same. To a doctor it has red blood
cells, and several types of white cells, plasma and other stuff like
maybe germs. Since it can be shown to have parts
inhomogeneous, but if you're looking at a bucket of it. Homogeneous.
It depends on the scale of observation
More obvious inhomogeneous mixtures would be like chocolate chip cookies, or trail mix. Ugh, leave out the raisins!! : o
 Inhomogeneous, pick out your favorite pieces.
Inhomogeneous, pick out your favorite pieces. Oil and water inhomogeneous mix.
Oil and water inhomogeneous mix.
Homogeneous mixture
 Drops of sea water all have
the same salinity. So they might be an example of a homogeneous mixture.
Drops of sea water all have
the same salinity. So they might be an example of a homogeneous mixture.
Every drop would have same ratio of Na ions, Cl ions and H2O
molecules. When every part is the same its homogeneous.
The air we breathe is also a homogeneous mixture. Each breath is 80% nitrogen and 20% oxygen.