Alright, so now you've reviewed a bit about matter, before we can make sense out of Chemistry, there's something you should know: Chemists are not real fond of magic! Uh, well, err... chemists generally think you can't get something for nothing. Certainly that's true in chemical reactions. You can only get the same number of the same type of atoms out of a reaction that you started with. No magic! Sorry : (

Its the Law!
The Law of Conservation of Mass
Matter cannot be created nor destroyed by normal chemical reactions. That means no disappearing of atoms, no pulling them out of a magicians hat either.

So you must balance equations. The must have the same number of the same atoms on the left and on the right of the arrow of an equation. We can't create or destroy atoms, we just rearrange partners.
Consider heating up potassium chlorate (a dangerous chemical) when heated it can break down to potassium chloride and oxygen because those atoms were present in the original compound. We can also write this equation:
2 KClO3 ---> 2 KCl + 3 O2
What this says in english is that 2 molecules of potassium chlorate will break down into two molecules of potassium chloride and 3 molecules of oxygen. It further shows us that a potassium chlorate molecule is made of 1 atom of potassium, 1 atom of chlorine, and 3 atoms of oxygen. Potassium chloride is made of 1 atom of potassium and 1 atom of chlorine. Oxygen molecules are made of two atoms of oxygen. (Strictly speaking both potassium chlorate and potassium chloride are ionic substances).
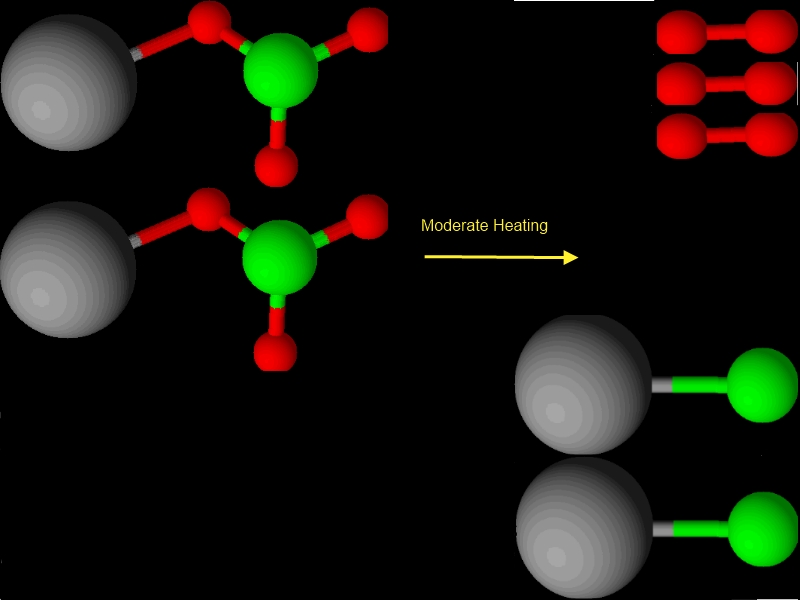
Grey = potassium atoms, red= oxygen atoms, green = chlorine atoms
Check out the balancing 2 K's left, 2 K's right
2 Cl's left, 2 Cl's right
6 O's left, 6 O's right. Alright!!!! Balanced! Yahoo!!!
2 KClO3 ---> 2 KCl + 3 O2
If you've still got time to kill. Search the internet for Gummy Bear and potassium chlorate :)