
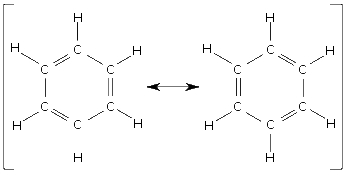
According to the IUPAC naming rules we would name the left structure cyclo (ring) hexa (6 carbons ) atriene (3 double bonds). We then give the double bonds the lowest possible addresses. Which would be cyclohexa-1,3,5-triene. Excellent job!! Ah... but there is a proverbial fly in the ointment!


The right structure would be cyclohexa-2,4,6-triene. Yet there is really no difference between the structures. Only the starting point. This molecule is both structures at the same time, or neither.
When 2 or more viable Lewis structures exist for a molecule it is said to posses resonance. Reality is some kind of fuzzy blend of these structures, or not!!! This is actually a major clue that our bonding models are sadly rather inadequate!
It is time for some fuzzy logic.

Well, blurry logic anyway. We typically draw this structure as a hexagonal ring with a circle in the middle. The circle in the middle represents the 3 double bonds that are distributed throughout the the ring. So if the IUPAC name doesn't really work, what are we do ??? So is it 3 double bonds and 3 single bonds. Or is it 6 'bond- and- a- halfs'???
Lets just agree to call it benzene most of the time.
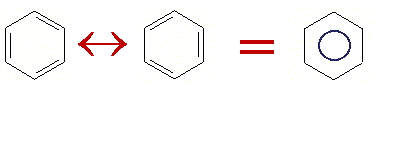
How to you blend distinct structures? Well perhaps we should recognize that electrons can do some amazing things, like delocalize. Pi bonds above and below the plane of the nuclei sort of merge. The bonding electrons no longer really belong to any particular nucleus. They are said to be delocalized. More on that later...
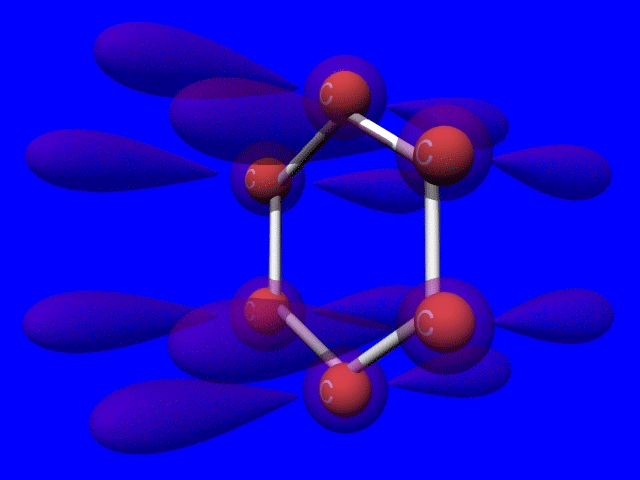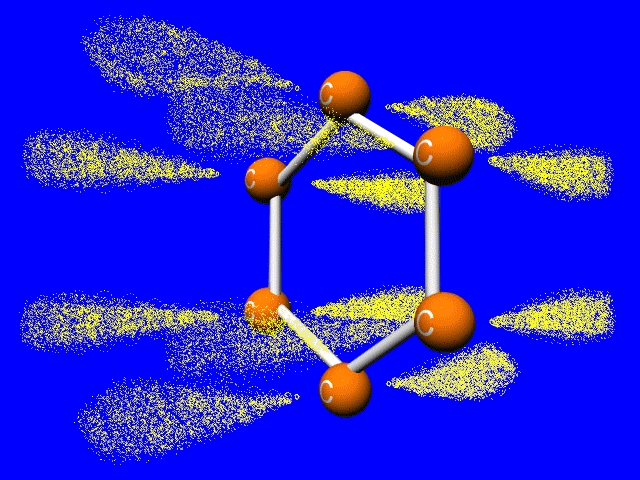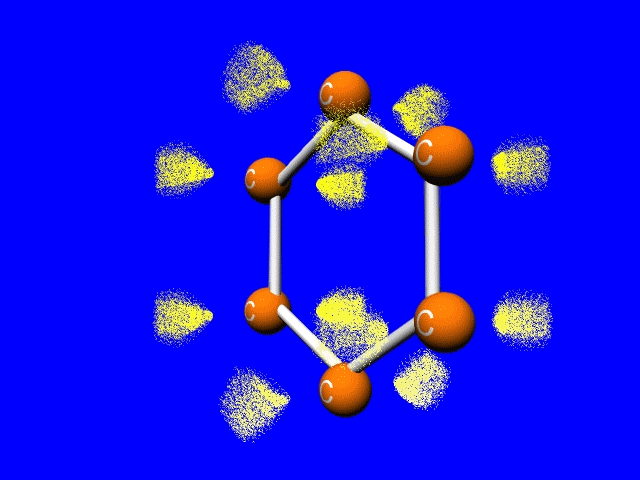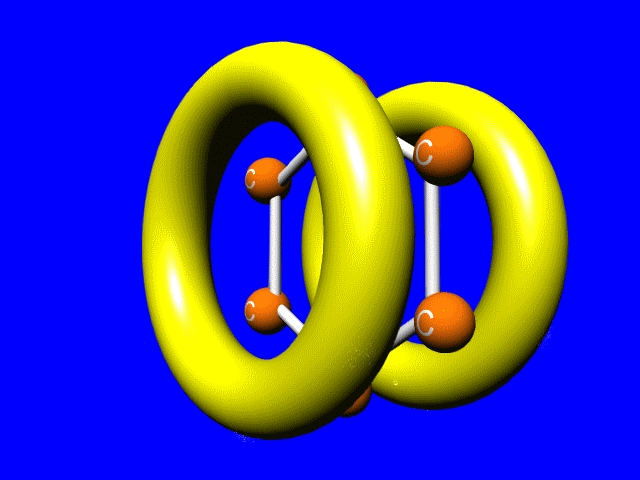
Original graphics by Tina Nye. Again Thanks to Tina Nye (class of 08) for her tireless work rendering graphics. Good Luck at University.
The jellybean like p-orbitals merge to form a delocalized set of doughnut like pi-bonds. Sigma bonds are shown by the white bars connecting the carbons.
Other structure with multiple bonds exhibit resonance, such as nitrate ion:
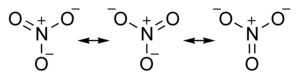
Turns out each bond has the same length. A little longer than a double bond, but shorter than a single bond. One and a third bond anyone?
The Lewis structure method is much quicker than rendering 3-d graphics and a double arrow ishow you show resonance with Lewis structures. Anytime you have more than one valid structure for a compound then that structure has resonance. That implies delocalized pi bonds.