
Original graphics art work by Tina Nye, Carson High School, class of 2008. Great Job !! Thank you for your hours of work, and your dedication to helping others learn. Mr. K.
As outlined on the page on Benzene, the IUPAC method of naming organic structures breaks down when we can't pick a single carbon and begin numbering from there. The resonance, or distributed nature of the double bonds messes us up there.
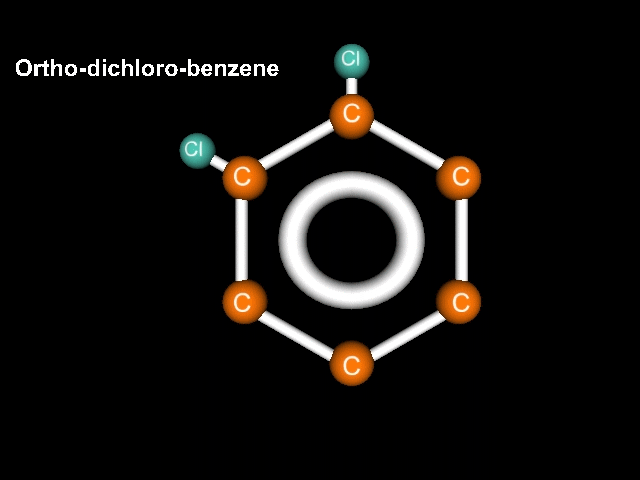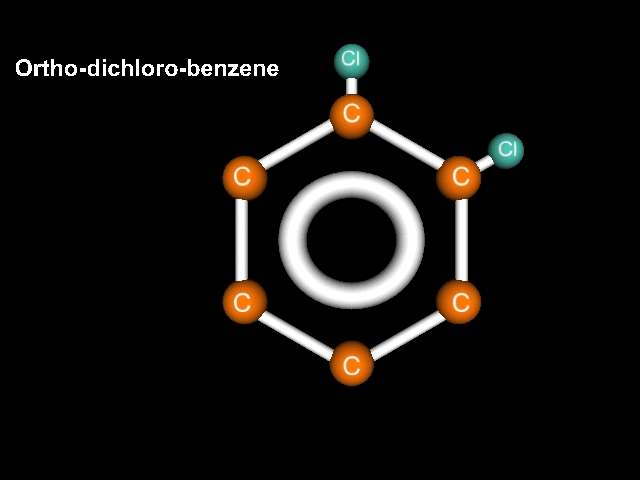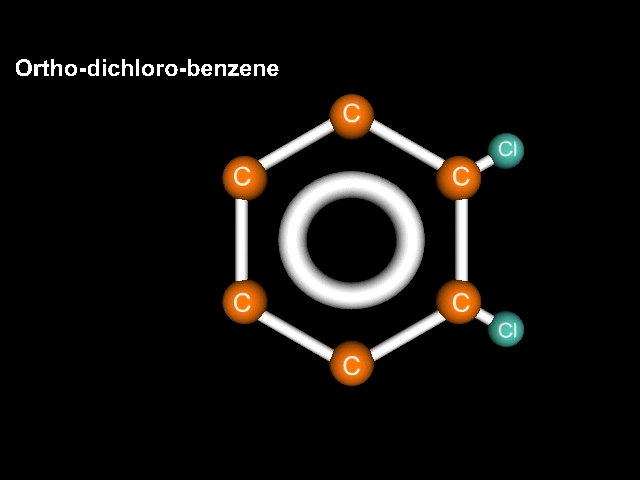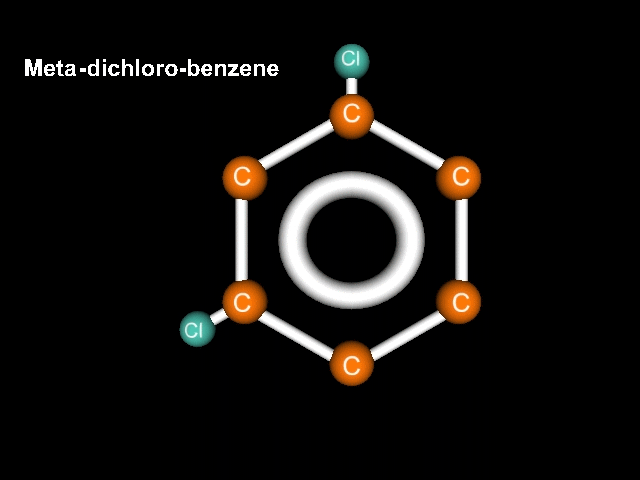
Consider a benzene molecule to which have been added a couple of halogen atoms. In this case lets use chlorine. If we have two chlorines, the name should reflect that. Let's try dichlorobenzene. There that should do it!
Ah, but Mr. K!!! What about the geometry? After all, think about it, the chlorines could be right next door to each other, could be completely across the structure from each other, or could be in a middle position!!! Well, what are we going to do about this???
As Tina so aptly illustrates above, we do give names based on the relative positions of the substituents.
If the atoms (substituents) are clOse we call it Ortho
If the atoms (substituents) are apart we call it para
If the atoms (substituents) are midway we call it meta
The substituents do not rotate around or migrate, but could be found in any of the 3 positions. Learn to tell them apart!
If the benzene is given two methyls, it is not called dimethyl benzene, it is because of longer tradition and other excuses (mostly just to be confusing) called xylene. And of course the methyls have to be described as Ortho, Meta, and Para depending on their geometry. Remember this one it is a teacher favorite so watch for it on tests.
Self test: O-xyleneM-dibromobenzene