
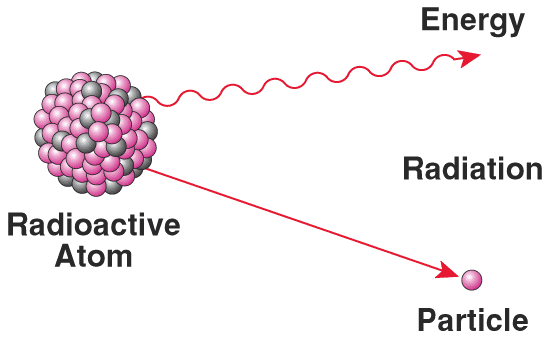

Radioactivity is the emission of particles and energy from an unstable nucleus to become stable. Sometimes it takes several steps. The shorter the half-life, the more energy and particles are emitted per unit of time; the more radioactive a substance is. Certain isotopes areare stable others unstable. Unstable isotopes may have too many neutrons or not enough neutrons to fully stabilize a nucleus. All isotopes of all elements 84 (Po) and above are unstable (radioactive).
Understanding the Nuclear Symbol
Atomic mass (protons + neutrons)
23592U
Atomic number (number of protons)
This is Uranium-235 a special isotope of uranium that can fission. It is used in nuclear fuel rods. Uranium-239 is way more common, it is radioactive, but will not produce a nuclear fission reaction. We need to know exactly what isotope we are working with.
Common ionizing radiation includes
| alpha | 42 He | Helium nucleus |
| beta | 0-1e | electron from nucleus |
| gamma | ultra high energy photons | |
| positron | 0+1e | positively charged electron |
| neutron | 10n | neutron ejected from nucleus |
Examples of nuclear decay reactions
Key concept 1: Nuclear decay reactions must obey the Law of Conservation of Mass.
Key concept 2: Nuclear decay reactions obey the Law of Conservation of Charge.
Put simply, this means the products have to add up to equal the decaying reactant (parent nuclide or isotope.) We need to pay attention to charges and to total mass.
alpha decay
23592U ==> 42 He + 23190Th
beta decay
146C ==> 0-1e + 147N
positron decay
2211Na ==> 0+1e + 2210Ne
fusion reaction with neutron emission
21H + 31H ==> 42He + 10
Each of these reactions are also accompanied by the release of energy as gamma rays (high energy photons).
The energy release is sufficient to excite the electrons in anything they hit so much that the electrons leave the atom. This leaves behind an ion. Hence the name ionizing radiation. If we turn very many atoms in your body to ions, cell damage occurs. This can lead to sickness and death. If the cells affected are sex cells, the damage can lead to mutation. For more on the characteristics of each radiation type check here.