
Is Dr. H2O2F2Br2I2N2Cl2 in the house??
There are 7 diatomic molecular elements on the periodic table. When they are in the elemental state (all by themselves, not tied up in a compound) they must have a subscript 2 behind them.
Each molecule is made of two atoms of the substance.
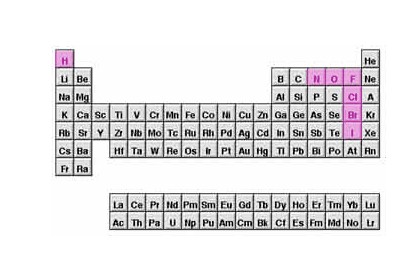
I like to think of them as the 7 hip-hop stars of the Periodic Table. Sure... they only come in Two-packs [Tupacs !!!! ; ) ]
Tupac Hydrogen, Tupac Oxygen, Tupac Nitrogen, Tupac Fluorine, Tupac Chlorine, Tupac Bromine, Tupac Iodine. Oh Yeah!
They never exist as single atoms. They are either tied up in a compound, or tied up (chemically bonded) with each other as two packs (diatomic molecules). When balancing equations, you can never have one of these seven elements all by itself as a single atom. Doesn't happen. They can be in a compound however without the subscript two. Once they are in a compound they follow the criss-cross rules.
Example: Suppose we tried to react aluminum with oxygen to make aluminum oxide.
- First write a skeleton equation:
Al + O ---> AlO - Make sure compounds are correct using diatomic
rules, criss-cross and Greek prefixes as needed.
Al + O2 ---> Al2O3 - Now balance left to right making sure the totals for each element are the
same. Only change the coefficients in front of the compound or element now. Never
change subscripts once the compounds are correct.
4Al + 3O2 ---> 2Al2O3