
When reviewing for your final, do not neglect topics that have only been mentioned in passing. These are important topics that sometimes get neglected due to time constraints, lost days, etc. : )
VSEPR Theory: Molecular geometry depending on how many electron pairs surround an atom. Don't forget about lone pairs, they help define the geometry, but are not included in the molecular shape itself. So the H20 molecule is bent at almost 109 degrees but is not tetrahedral.
Empirical Formulas: Calculating a chemical formula from weight %. Sort of related to calculating weight percents by using a formula and GFW's only in reverse
Sigma and Pi bonding: When two atoms get close enough to bond, their electron orbitals are hybridized. When 2-s orbital overlap the result is a sigma bond. If two p's overlap end to end the result is a sigma bond. If additional bonds occur they are p's lengthwise which result in a pi bond. Pi bonds are weaker. Rule of Thumb: all single bonds are sigma bonds. The 1st bond of a multiple bond is also a sigma, the second and/or third bond of a multiple bond are pi bonds.
Example: consider ethene. 5 sigma bonds 1 pi bond: All of the H-C bonds are singles. 1 of the C-C bonds is sigma and 1 of C-C bonds (the second) is a pi bond
Composition of the atmosphere: scroll down a bit look for pie diagram.
Limiting reagent: A reaction typically stops when you run out of something. Whichever reactant runs out first limits the reaction and is called the limiting reagent. If you wanted to make Hamburgers according to the following recipe: 1 patty 2 buns 1 tomato 1 pickle.
How many hamburgers could you make with:
6 patties
14 buns
7 pickles
8 tomato slices
What is the limiting reagent?
In a numerical chemical approach given two or more reactants. Do a mass-mass stoichiometry problem with the given amount of each chemical. Which ever is least will be true because it is the limiting reagent.
Example: 3 Moles H2 and 2 Moles O2 are combined. How much water can be made?
Balanced Equation: 2H2 + O2 --> 2 H2O
Factor Label problem:
|
3 Moles H2 |
2 mol H2O | = 3.0 mol H2O |
| 2 mol H2 |
|
2 Moles O2 |
2 mol H2O | = 4.0 mol H2O |
| 1 mol O2 |
Answer: It is only possible to make 3.0 mol H2O and you will have 0.5 mol left-over O2
Kinetic energy and gases: Recall that gases at the same temperature and pressure must have the same kinetic energy. Since KE=1/2mv2 it follows that massive atoms will be moving slower than less massive atoms at the same temperature.
Colligative properties: These properties of solutions don't depend on what is being dissolved just the mole ratio. The colligative properties are freezing point depression, boiling point elevation, and vapor pressure decrease.
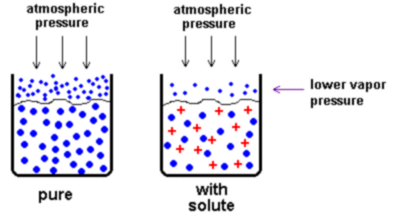
Let the red crosses symbolize a non-volatile (low vapor pressure) We can see that the solute will lower the vapor pressure of the solvent because after dissolving the solute takes the place of some of the atoms of solvent on the surface. Those "missing" atoms aren't pushing, so less vapor pressure : )
Conductivity: Soluble ionic substances conduct electricity when dissolved in water. Ionic bonding occurs between elements that are farther apart on the periodic table. Molecular substances (covalent bonds) do not conduct electricity.
Nuclear notation: 186O-2 what the heck to all those little numbers mean? Remember #neutrons = mass - protons.
Entropy: Entropy is a sense of disorder, freedom of motion, or randomness. Reactions that increase entropy (of the Universe) are spontaneous. They might include reactions or changes that produce: melting, heating, vaporizing, increase in volume, mixing, making of more number of moles than reactants, dissolving.
