
A second pass at Mr. K's famous cocoa problem. Take 250 mL of cocoa at 98 degrees C and add 25 grams of ice at 0 C. What is the final temperature of the cocoa?
Background knowledge:
Energy is not created nor destroyed in Thermo (or anywhere else).
Since Energy is not created nor destroyed then the heat lost by cooling the
cocoa will do two things: melt the ice
cube, and then warm the resulting water up to Tfinal
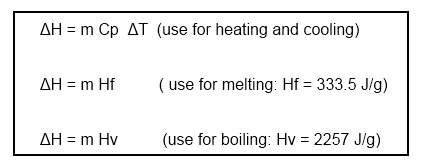
Important equations for calorimetry:
Adding ice to 98 C liquid causes ice to melt. This cools cocoa (which we will assume behaves like pure water)
Melting: ice travels from point 4 to point 5.
Enthalpy = m Hf (mass of ice x heat of fusion of ice)
25 g x 333.5 J/g = + 8337.5 J this must come from cocoa so it travels from point 1 to point 2
Enthalpy = m Cp DeltaT (mass of cocoa x heat capacity of cocoa ( use water) x Delta T)
-8337.5 = 250 g x 4.18 J/gºC x DeltaT
Delta t = -7.97 ºC so at this step our cocoa is
91ºC. Now the melted ice is at 5 and the cocoa is at 2. They must meet at
point 3. But where is this. Again the heat lost by the cocoa
will be gained by the melted ice.
m Cp delta T (of melted ice) = m Cp delta T (of cooling cocoa)
25 g x 4.18 J/gºC x (Tf - 0) = 250 g x 4.18 J/gºC x ( 91-Tf)
104.5 Tf = -1045 Tf - 95095
1149.5 Tf= 95095
Tf = 83 ºC and hopefully ready to drink.
Try it at home and tell us how close Thermodynamics actually matches the real
world.