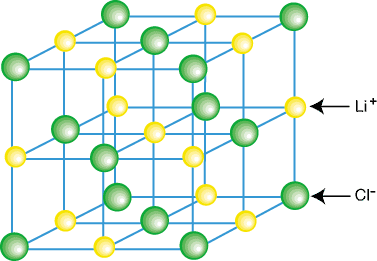
Ionic substances form 3-d arrays of alternating cations and anions that charge balance each other. This one is called face-centered cubic.
To calculate the lattice energy we must think of all the steps necessary to make a crystal from it's elements. This is the amount of energy given off when 1 mole of the solid is produced.
We will use Hess's Law to rearrange the steps so that we get the net equation we are after. We will sum the energies of each step and that will be our total lattice energy. Remember if you flip an equation, you must reverse its sign. If you multiply an equation remember to multiply the corresponding energy.
Example: Calculate the lattice energy of LiBr given the following
| Atomization energy Br2 | 111.7 kJ/mol |
| Ionization energy Li | 520.0 kJ/mol |
| Vaporization energy Br | 15.46 kJ/mol |
| Vaporization energy Li | 134.7 kJ/mol |
| Ionize Br2 | -324.0 kJ/mol |
| Form LiBr | -351.2 kJ/mol |
Write an equation for each energy step. I like to order the steps in a possible sequence.
| Vaporize Li | Li (s) --> Li (g) | 134.7 kJ/mol |
| Vaporize Br2 | Br2(l) --> Br2(g) | 15.46 kJ/mol |
| Atomize Br2 | Br2 --> 2Br | 111.7 kJ/mol |
| Ionize Li | Li --> Li+ +e- | 520.0 kJ/mol |
| Ionize Br | Br +e- --> Br- |
-324.0 kJ/mol |
| Form LiBr | Li(s) + 1/2 Br2(l) --> LiBr(s) | -351.2 kJ/mol |
Now we re-arrange these steps so that we get a net equation for:
Li+ + Br- --> LiBr(s)
| flip Vaporize Li | Li(g) --> Li (s) | -134.7 kJ/mol |
| flip Vaporize Br2 | (1/2) Br2(g) --> Br2(l) | (1/2) -15.46 kJ/mol |
| flip Atomize Br2 | (1/2) 2Br --> Br2 | (1/2) -111.7 kJ/mol |
| flip Ionize Li | Li+ +e- --> Li | -520.0 kJ/mol |
| Ionize Br | Br +e- --> Br- |
-324.0 kJ/mol |
| Form LiBr | Li(s) + 1/2 Br2(l) --> LiBr(s) | -351.2 kJ/mol |
Check cancellation to get desired net equation
Li+ + Br- --> LiBr(s)
| Vaporize Li | -134.7 kJ/mol | |
| Vaporize Br2 | (1/2) |
(1/2) -15.46 kJ/mol |
| Atomize Br2 | (1/2) |
(1/2) -111.7 kJ/mol |
| Ionize Li | Li+ +e- --> |
-520.0 kJ/mol |
| Ionize Br | Br- --> |
-324.0 kJ/mol |
| Form LiBr | -351.2 kJ/mol | |
| Lattice energy | -1393.48 kJ/mole |
Li+ + Br- --> LiBr(s)