Water is an amazing compound. It is one of the key ingredients of life, an almost universal solvent, extra high heat capacity etc.

One of the most amazing feats of water is that it sometimes acts as an acid, but it can also act like a base. It can even donate a proton to itself, and it can accept the proton.
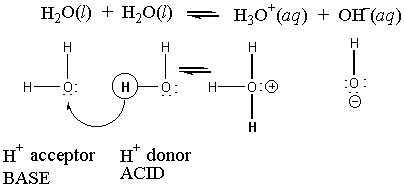
This is the autoionization of water. The equilibrium expression for this reaction would be:
[H3O+][OH-]
[H2O][H2O]
Remember to leave out pure liquids from Keq expressions.
[H3O+][OH-] = Keq of water or Kw
Kw = 10-14 always that's why it called constant!