
The key to this lab is: if Charles Law is really true, then the only thing controlling the volume of a gas is the temperature. So big jump here... if the temperature were to drop to zero (absolute), what would the volume do......?
Well, it would have to drop to zero also! (When a gas matches our mathematical models it is called ideal.)
We can use this relationship (Charles Law) to measure how much shrinking we get for how much cooling. By carefully graphing this data in lab over conditions that we can achieve we can extend the graph backwards to the theoretical point where volume would equal 0. If the volume of a gas is zero, it must be at absolute zero!
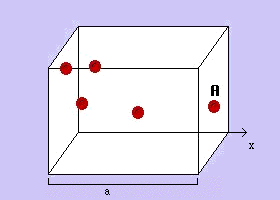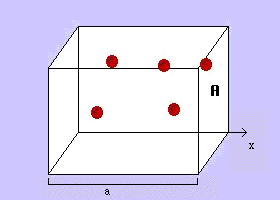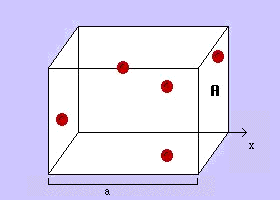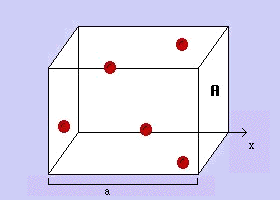
The graph you make should look something like:
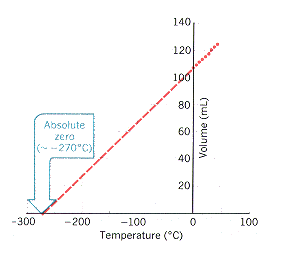
Remember: graph our 3 data points using Excel or Google sheets, then extend them backwards to volume = 0 (the x intercept).
Note: we are working in degrees C we get the C value of 0 absolute. We also have an independent estimate of the C to K conversion!