Anything but the boiling water.....

Heeelppp meee I'm meeellllllllting !!!!
Step 1:Calibrate the thermometers
Phase changes are chosen because they occur at known temperatures.
Ice water slurry: 0º C
Boiling water: depends on the
barometric pressure.
Our lab averages between 637 mm Hg and 640 mm Hg.
This corresponds to a boiling point of approximately 95.25 °C
Create a data table
| Phase change | Known temperature. | Thermometer reading |
| ice/water | ||
| water/steam | ||
| ethanol/vapor |
Plot these data and connect a smooth line.
Sample graph only (cleverly faked by Mr. K) Do not use in real lab reports. Do make yours the same way! ;)
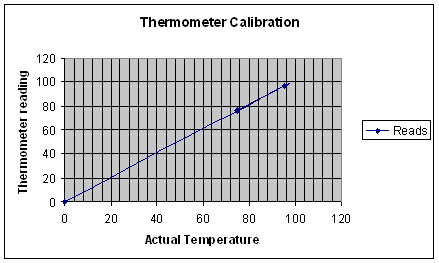
You will want to make your as big as possible to make it easy to read your correlation graph for the other parts of this lab. When you are determining the boiling points of unknowns, plot the reading on the axis, project horizontally until you intersect the line. Drop a perpendicular to the X-axis and use this number as the true boiling point determination.
Step 2: Determine the boiling point of Unknown A and Unknown B using the set up in your lab hand-out.
Slow heating and multiple trials will add data consistency.
Write your steps and data table into your Lab notebook. After it is drawn, you should trim and paste your correlation graph into the lab notebook as well.
 Cool microphoto of a solid beginning to melt!
Cool microphoto of a solid beginning to melt!
Your two substances A and B were 2-butanol at _____ C boiling point, and acetone at _________ C boiling point.
When determining boiling point:
Did you remember to take at least 3 trials that agreed with each other?
Did you remember to read the corrected value from your thermometer correlation?
If you tried to identify your unknowns by looking up the boiling points in a reference, did you think to correct for sea level. As an approximation you could add back the difference between ethanol at room pressure and ethanol at standard pressure. It was about 4 degrees.
Some chemical theory:
What is the effect of increasing the molecular weight of a liquid on its boiling point? In general the higher the molecular weight, the higher the boiling point for organics.
This rule of thumb must be modified for each organic family. Alcohols with the OH functional group can form hydrogen bonds with each other so alcohols in general have higher boiling points than other organics of similar molecular mass. Ketones (the family that acetone belongs to cannot hydrogen bond to themselves so have considerably lower boiling points.
Water is so polar and the hydrogen bonding is so prevalent that it boils at 100 ºC (Std Pressure) even though its molar mass is only 18.02 grams!
Composition: Given a mix of A and B, the composition of the mixture will be proportional to the distance the boiling point is from A compared to the total distance. In other words, if the melting point was halfway between A and B your composition is 50%A, 50%B