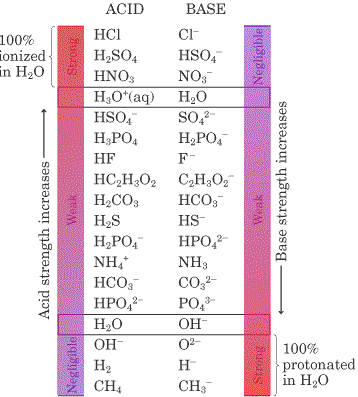
The inverse relationship between the strength of an acid and its conjugate base may seem a bit counter-intuitive at first, but give it some thought.
If the base (proton acceptor) really wants to keep the proton, the molecule can't dissociate and ionize. This means if you have a strong base, you must have a weak acid.
1. The conjugate base of a strong acid has negligible affinity for protons. It is a very weak conjugate base.
2. A weak acid only partially ionizes in water. It's conjugate base has considerable affinity for protons. It wants to stay as a molecule.
3. The strongest conjugate bases belong to chemicals such a CH4 and H2 that have H but hardly ever give up a proton. If they ever do they will steal the proton back from water immediately.
Think of a tug of war
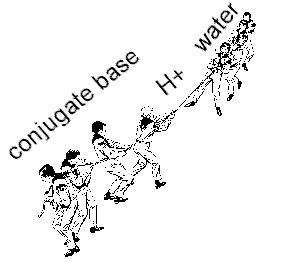
If the conjugate base is stronger than water, it ends up with the proton. Hydroxide is produced pH goes up: CB- + H2O --> HCB + OH-
If water is the stronger conjugate base, then water gets the proton and a hydronium is produced. pH goes down.: HCB + H20 --> CB- + H3O+
Consider the following reaction:
HSO4-(aq) + CO32-(aq)<--> SO42-(aq) + HCO3- (aq)
Predict whether this reaction would tend to run towards the right (forward, Keq> 1) or toward the left (backward, Keq <1).
Find CO32- and SO42- on the Base side of the diagram at the top of the page. Since CO32- is the stronger conjugate base, it takes the proton and the equation runs to the right. It would have a Keq > 1.
Nifty Huh!!!!