Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
__________ is the chemical symbol for elemental sodium.
|
|
|
2.
|
The symbol for the element potassium is __________.
|
|
|
3.
|
The temperature of 25°C is __________ in Kelvins.
a. | 103 | b. | 138 | c. | 166 | d. | 248 | e. | 298 |
|
|
|
4.
|
The freezing point of water at 1 atm pressure is __________.
a. | 0°F | b. | 0 K | c. | 0°C | d. | –273°C | e. | –32°F |
|
|
|
5.
|
1 kilogram = __________ milligrams
a. | 1 ´ 10–6 | b. | 1,000 | c. | 10,000 | d. | 1,000,000 | e. | none of the
above |
|
|
|
6.
|
"Absolute zero" refers to __________.
a. | 0 Kelvin | b. | 0° Fahrenheit | c. | 0°
Celsius | d. | °C + 9/5(°F – 32) | e. | 273.15°C |
|
|
|
7.
|
The density (in g/cm3) of a gold nugget that has a volume of 1.68
cm3 and a mass of 32.4 g is __________.
a. | 0.0519 | b. | 19.3 | c. | 54.4 | d. | 0.0184 | e. | 32.4 |
|
|
|
8.
|
The number 0.00430 has __________ significant figures.
|
|
|
9.
|
The correct result (indicating the proper number of significant figures)
of the following addition is __________.
12
1.2
0.12
+ 0.012
a. | 13 | b. | 13.3 | c. | 13.33 | d. | 13.332 | e. | none of the
above |
|
|
|
10.
|
There should be __________ significant figures in the answer to the following
computation. 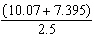
|
|
|
11.
|
Which one of the following is a pure substance?
a. | concrete | b. | wood | c. | salt
water | d. | elemental copper | e. | milk |
|
|
|
12.
|
Gases and liquids share the property of __________.
a. | compressibility | b. | definite volume | c. | incompressibility | d. | indefinite shape | e. | definite
shape |
|
|
|
13.
|
A certain mass of carbon reacts with 13.6 g of oxygen to form carbon monoxide.
__________ grams of oxygen would react with that same mass of carbon to form carbon dioxide,
according to the law of multiple proportions?
a. | 25.6 | b. | 6.8 | c. | 13.6 | d. | 136 | e. | 27.2 |
|
|
|
14.
|
Which statement below correctly describes the responses of alpha, beta, and
gamma radiation to an electric field?
a. | Both beta and gamma are deflected in the same direction, while alpha shows no
response. | b. | Both alpha and gamma are deflected in the same direction, while beta shows no
response. | c. | Both alpha and beta are deflected in the same direction, while gamma shows no
response. | d. | Alpha and beta are deflected in opposite directions, while gamma shows no
response. | e. | Only alpha is deflected, while beta and gamma show no
response. |
|
|
|
15.
|
__________ and __________ reside in the atomic nucleus.
a. | Protons, electrons | b. | Electrons, neutrons | c. | Protons,
neutrons | d. | none of the above | e. | Neutrons, only
neutrons |
|
|
|
16.
|
Which pair of atoms constitutes a pair of isotopes of the same element?
|
|
|
17.
|
The element __________ is the most similar to strontium in chemical and physical
properties.
|
|
|
18.
|
The empirical formula of a compound with molecules containing 12 carbon atoms,
14 hydrogen atoms, and 6 oxygen atoms is __________.
a. | C12H14O6 | b. | CHO | c. | CH2O | d. | C6H7O3 | e. | C2H4O |
|
|
|
19.
|
What is the formula of the compound formed between strontium ions and nitrogen
ions?
a. | SrN | b. | Sr3N2 | c. | Sr2N3 | d. | SrN2 | e. | SrN3 |
|
|
|
20.
|
Magnesium reacts with a certain element to form a compound with the general
formula MgX. What would the most likely formula be for the compound formed between potassium
and element X?
a. | K2X | b. | KX2 | c. | K2X3 | d. | K2X2 | e. | KX |
|
|
|
21.
|
The correct name for K2S is __________.
a. | potassium sulfate | b. | potassium disulfide | c. | potassium
bisulfide | d. | potassium sulfide | e. | dipotassium
sulfate |
|
|
|
22.
|
The correct name for Al2O3 is __________.
a. | aluminum oxide | b. | dialuminum oxide | c. | dialuminum
trioxide | d. | aluminum hydroxide | e. | aluminum
trioxide |
|
|
|
23.
|
The correct name for SO is __________.
a. | sulfur oxide | b. | sulfur monoxide | c. | sulfoxide | d. | sulfate | e. | sulfite |
|
|
|
24.
|
The formula of ammonium carbonate is __________.
a. | (NH4)2CO3 | b. | NH4CO2 | c. | (NH3)2CO4 | d. | (NH3)2CO3 | e. | N2(CO3)3 |
|
|
|
25.
|
The correct name for Mg(ClO3)2 is __________.
a. | magnesium chlorate | b. | manganese chlorate | c. | magnesium
chloroxide | d. | magnesium perchlorate | e. | manganese
perchlorate |
|
|
|
26.
|
Chromium and chlorine form an ionic compound whose formula is
CrCl3. The name of this compound is __________.
a. | chromium chlorine | b. | chromium(III) chloride | c. | monochromium
trichloride | d. | chromium(III) trichloride | e. | chromic
trichloride |
|
|
|
27.
|
When the following equation is balanced, the coefficients are __________.
NH3 (g) + O2 (g) ®
NO2 (g) + H2O (g)
a. | 1, 1, 1, 1 | b. | 4, 7, 4, 6 | c. | 2, 3, 2,
3 | d. | 1, 3, 1, 2 | e. | 4, 3, 4, 3 |
|
|
|
28.
|
When the following equation is balanced, the coefficient of H2S is
__________.
FeCl3 (aq) + H2S (g) ® Fe2S3 (s) + HCl (aq)
|
|
|
29.
|
How many grams of hydrogen are in 46 g of CH4O?
a. | 5.8 | b. | 1.5 | c. | 2.8 | d. | 0.36 | e. | 184 |
|
|
|
30.
|
A 22.5-g sample of ammonium carbonate contains __________ mol of ammonium
ions.
a. | 0.468 | b. | 0.288 | c. | 0.234 | d. | 2.14 | e. | 3.47 |
|
|
|
31.
|
What is the empirical formula of a compound that contains 27.0% S, 13.4% O, and
59.6% Cl by mass?
a. | SOCl | b. | SOCl2 | c. | S2OCl | d. | SO2Cl | e. | ClSO4 |
|
|
|
32.
|
Magnesium and nitrogen react in a combination reaction to produce magnesium
nitride:
3 Mg + N2 ®
Mg3N2
In a particular experiment, a 9.27-g sample of N2
reacts completely. The mass of Mg consumed is __________ g.
a. | 8.04 | b. | 24.1 | c. | 16.1 | d. | 0.92 | e. | 13.9 |
|
|
|
33.
|
The combustion of ammonia in the presence of excess oxygen yields NO2
and H2O:
4 NH3 (g) + 7 O2 (g) ® 4 NO2 (g) + 6 H2O (g)
The
combustion of 28.8 g of ammonia consumes __________ g of oxygen.
a. | 94.7 | b. | 54.1 | c. | 108 | d. | 15.3 | e. | 28.8 |
|
|
|
34.
|
The combustion of propane (C3H8) produces CO2
and H2O:
C3H8 (g) + 5O2 (g) ® 3CO2 (g) + 4H2O (g)
The
reaction of 2.5 mol of O2 will produce __________ mol of H2O.
a. | 4.0 | b. | 3.0 | c. | 2.5 | d. | 2.0 | e. | 1.0 |
|
|
|
35.
|
Lead (II) carbonate decomposes to give lead (II) oxide and carbon
dioxide:
PbCO3 (s) ® PbO (s) +
CO2 (g)
How many grams of lead (II) oxide will be produced by the decomposition of
2.50 g of lead (II) carbonate?
a. | 0.41 | b. | 2.50 | c. | 0.00936 | d. | 2.09 | e. | 2.61 |
|