Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is the symbol for phosphorus
a. | Po | d. | K | b. | Ph | e. | none of the above | c. | P |
|
|
|
2.
|
What is the formula for iron (III) bromide
a. | Fe3B | d. | IB | b. | FeBr3 | e. | IrBr3 | c. | Ir3Br |
|
|
|
3.
|
What is the charge on a Beryllium ion
|
|
|
4.
|
What is the formula for sodium hydroxide
a. | SoOH | d. | Na(OH)2 | b. | NaOH | e. | SHO2 | c. | SOH |
|
|
|
5.
|
What is the Name for PO3
a. | phosphite | d. | phosphorus trioxide | b. | trioxyphosphide | e. | phosphorus (II) oxyate | c. | phosphorus
oxide |
|
|
|
6.
|
What is the name for Cr2O3
a. | dichromate trixoxide | d. | chrome oxide | b. | chromium (II) trioxide | e. | dichrome polyoxide | c. | chromium (III)
oxide |
|
|
|
7.
|
Which of the following is a metal
a. | calcium | d. | phosphate | b. | bromine | e. | germanium | c. | oxide |
|
|
|
8.
|
Which of the following describes nitrogen in its free state (or elemental
state)
a. | diatomic | d. | a & c only | b. | gas | e. | a, b & c | c. | non-metal |
|
|
|
9.
|
Which element would have chemical properties similar to oxygen?
a. | nitrogen | d. | boron | b. | fluorine | e. | argon | c. | sulfur |
|
|
|
10.
|
What is the correct formula for phosphoric acid
a. | HP | d. | HPO3 | b. | PH3 | e. | H3PO4 | c. | HPO4 |
|
|
|
11.
|
An empty graduated cylinder’s mass is 55.0 grams. Adding 20.0 mL of an
unknown liquid increases the mass to 105.00 grams. What is the density of the liquid
a. | 2.5 g/mL | d. | 5.25 g/mL | b. | 0.4 g/mL | e. | 0.52 g/mL | c. | 2.75
g/mL |
|
|
|
12.
|
When the equation C5H12 + O2 --> CO2
+ H2O is balanced, what is the coefficient in front of the O2?
a. | 3 | d. | 8 | b. | 6 | e. | cannot be balanced | c. | 12 |
|
|
|
13.
|
When the equation Fe(OH)3 ---> Fe2O3 +
H2O is balanced, the coefficient in front of the H2O is ?
a. | 3 | d. | 6 | b. | 2 | e. | cannot be balanced | c. | 1 |
|
|
|
14.
|
When the equation N2 + H2 --> NH3 is
balanced, the coefficient in front of the H2 is?
a. | 4 | d. | 1 | b. | 2 | e. | cannot be balanced | c. | 3 |
|
|
|
15.
|
What is the coefficient in front of the CoCl2, when the
following reaction is completed and balanced.
CoCl2 +
Na3PO4 --->
|
|
|
16.
|
Which of the following when moderately heated, yields O2 as a product
a. | NaOH | d. | Na3PO4 | b. | NaClO3 | e. | NaHCO3 | c. | Na2CrO4 |
|
|
|
17.
|
What is the molar mass (also known as gram formula weight) of
(NH4)2SO4
a. | 132.1 g | d. | 264.1 g | b. | 66.1 g | e. | 156.1 g | c. | 63.1 g
|
|
|
|
18.
|
How many Nitrogen atoms are there in 2.8 grams of N2
a. | 6.02 x 10 23 atoms | d. | 28 atoms | b. | 6.02 x 10
22 atoms | e. | 14.01 x
10 23 atoms | c. | 1.20 x 10 23
atoms |
|
|
|
19.
|
What is the mass of 0.100 moles of water?
a. | 16.00 grams | d. | 6.02 x 10 23 grams | b. | 18.0 grams
| e. | none of the
above | c. | 1.8 grams |
|
|
|
20.
|
A certain gas has a volume of 10.01 Liters at STP and is found to mass 14.3
grams. What is its molar mass (also known as molecular weight)?
a. | 32.0 grams | d. | 2.02 grams | b. | 14.0 grams | e. | 20.19 grams | c. | 28.0 grams
|
|
|
|
21.
|
What is the density of a rectangular piece of metal that measures 2 cm x 3 cm x
4 cm and has a mass of 120 grams
a. | 3.0 g/cm3 | d. | 12.0 g/ cm3 | b. | 5.0
g/cm3 | e. | none of
the above | c. | 0.2 g/ cm3 |
|
|
|
22.
|
How many mm in 0.000187 Km
a. | 187 mm | d. | 1.87 mm | b. | 18.7 mm | e. | none of the above | c. | 1.87 x 10 - 6
mm |
|
|
|
23.
|
How many significant digits in 0.005410
|
|
|
24.
|
40 + 10.0 + 1.5 = ? When significant digits are taken into account.
|
|
|
25.
|
6.2/3.80 = ? Round to correct number of significant digits.
a. | 2 | d. | 1.60 | b. | 1.6 | e. | none of the above | c. | 1.63 |
|
|
|
26.
|
A skier travels downhill for 8.3 minutes at a speed of 10.0 m/s, How far did she
travel in miles. Given 1 mi = 5280 ft. 1 ft = 0.305 m
a. | 3.09 mi | d. | 10.0 mi | b. | 0.29 mi | e. | 16.3 km | c. | 0.05
mi |
|
|
|
27.
|
How many grams of Fe would be needed to exactly react with 16.0 g
O2 to make iron (III) oxide ?
4 Fe + ____ O2 ---> 2
Fe2O3 (Balance the equation 1st)
a. | 55.85 g Fe | d. | 223.4 g Fe | b. | 37.2 g Fe | e. | 16.0 g Fe | c. | 64. g
Fe |
|
|
|
28.
|
What volume of CO2 (at STP) can be made with 62.0 grams of
H2CO3 if it decomposes according to:
H2CO3 --->
H2O + CO2
a. | 62.0 g | d. | 760 mL | b. | 62.0 mL | e. | 1 quart | c. | 22.4
L |
|
|
|
29.
|
How many grams of carbon would be needed to produce exactly 54.93 g of Mn from
MnO2 if it reacts according to: MnO2 + 2C --> Mn + 2 CO
a. | 2.0 g | d. | 54.93 g | b. | 12. 01 g | e. | 109 g | c. | 24.02
g |
|
|
|
30.
|
How much chlorine is needed to just react with 50.0 grams of MgBr2
?
MgBr2 + Cl2 --> MgCl2 + Br2
a. | 70.90 g | d. | 9.63 g | b. | 50.0 g | e. | 35.45 g | c. | 19.25 g
|
|
|
|
31.
|
What volume of H2 would be needed to make 36 grams of H2O
Assume STP.
a. | 44.8 L | d. | 6.02 x 10 23 atoms | b. | 22.4 L | e. | none of the above | c. | 2.02
g |
|
|
|
32.
|
10.0 Liters of gas at 20.0 C is heated to 60.0 C what is its new volume
a. | 10.0 L | d. | 30.0 L | b. | 11.4 L | e. | none of the above | c. | 20.0
L |
|
|
|
33.
|
What is the new temperature if 3.00 L of Argon gas that is originally at
200 K is heated until the volume is 4.50 L?
a. | 300 C | d. | 300 K | b. | 573 C | e. | none of the above | c. | 573
K |
|
|
|
34.
|
10.0 L of gas at 25.0 C is heated to 50.0 C what is its new volume?
a. | 20.0 L | d. | 5.0 L | b. | 35.0 L | e. | none of the above | c. | 10.8
L |
|
|
|
35.
|
600 cm3 of gas is at 300 mm Hg pressure, if the pressure increases to
900 mm Hg, what is the new volume of the gas?
a. | 900 cm3 | d. | 200 cm3 | b. | 600 cm3 | e. | 100 cm3 | c. | 300
cm3 |
|
|
|
36.
|
1.00 L of gas at STP is changed to 29 C and 642 mm Hg. What is the new
volume?
a. | 1.31 L | d. | 2.29 L | b. | 0.13 L | e. | none of the above | c. | 0.93
L |
|
|
|
37.
|
A chemical reaction generates 14.0 grams of NO2 gas at 640 mm Hg and
24 C. How many liters of volume are there? (R= 0.0821 )
a. | 9.21 L | d. | 14.0 L | b. | 8.51 L | e. | none of the above | c. | 9.79 x 10 -4
L |
|
|
|
38.
|
What is the correct Lewis structure for hydrogen chloride, HCl? 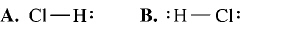 
|
|
|
39.
|
How many neutrons are there in 24Na
a. | 24 | d. | 11 | b. | 22.99 | e. | none of the choices | c. | 13 |
|
|
|
40.
|
What is the shape of the 3s suborbitals
a. | circular | d. | clover-leaf | b. | spherical | e. | ring | c. | dumbell
shaped |
|
|
|
41.
|
Which of the following represents and isotope rather than a typical atom.
a. | 16 O | d. | 65Zn | b. | 32 S | e. | 1H | c. | 33
P |
|
|
|
42.
|
The electron configuration of nitrogen is 1s2
2s2 2p3. How many more electrons does nitrogen need to satisfy
the octet rule?
|
|
|
43.
|
[Ar]4s23d104p3 is the electron
configuration of a(n) __________ atom.
|
|
|
44.
|
Which of the following atoms or ions most likely has the largest radius
|
|
|
45.
|
The amount of energy required to condense a gas to a liquid is known as
a. | Heat of vaporization | d. | Temperature | b. | Heat of fusion | e. | Heat of confusion | c. | Heat
capacity |
|
|
|
46.
|
What is the resulting energy change of the system when 75 grams of ice
melts?
a. | +6000 cal | d. | -75.0 cal | b. | -6000 cal | e. | 540 cal/joule | c. | +75.0
cal |
|
|
|
47.
|
How much heat is needed to melt a 25 gram ice cube and then have it boil at 100
C
a. | +18000 cal | d. | 1.00 cal/g °C | b. | 540 cal | e. | 80 cal/g | c. | 4500
cal |
|
|
|
48.
|
Sulfur dioxide can oxidize in air forming sulfur trioxide according to the
following chemical reaction; 2 SO 2 + O 2 --> 2 SO 3
calculate the !H rxn given the following thermodynamic
data Chemical | Heat of Formation | O2 | 0.00 k
cal/mol | SO2 | - 70.75
kcal/mol | SO3 | -94.46
kcal/mol | | |
a. | - 47.42 kcal | d. | -165.2 kcal | b. | -23.71 kcal | e. | none of the above | c. | +165.2
kcal |
|
|
|
49.
|
The reaction of 2 SO2 + O2 --> 2 SO3
is
a. | endothermic | d. | biothermic | b. | exothermic | e. | none of the above | c. | neither |
|
|
|
50.
|
Chemistry rocks because
a. | I do | d. | Science does | b. | My teachers do | e. | All of the choices are true :
) | c. | My school does |
|