Multiple Choice
Identify the choice that best completes the statement or answers the question.
Identify the choice that best completes the statement or answers the question.
1.
The value of Keq for the equilibrium
H2 (g) + I2 (g)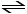 2 HI (g)
2 HI (g)
is 794 at 25°C. What is the value of Keq for the equilibrium below?
1/2 H2 (g) + 1/2 I2 (g)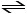 HI (g)
HI (g)
H2 (g) + I2 (g)
is 794 at 25°C. What is the value of Keq for the equilibrium below?
1/2 H2 (g) + 1/2 I2 (g)
a. | 397 |
b. | 0.035 |
c. | 28 |
d. | 1588 |
e. | 0.0013 |
2.
The value of Keq for the equilibrium
H2 (g) + I2 (g)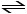 2 HI (g)
2 HI (g)
is 794 at 25°C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g)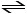 1/2 H2 (g) + 1/2 I2 (g)
1/2 H2 (g) + 1/2 I2 (g)
H2 (g) + I2 (g)
is 794 at 25°C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g)
a. | 1588 |
b. | 28 |
c. | 397 |
d. | 0.035 |
e. | 0.0013 |
3.
At elevated temperatures, molecular hydrogen and molecular bromine react to
partially form hydrogen bromide:
H2 (g) + Br2 (g)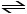 2HBr (g)
2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are __________ mol of Br2 present in the reaction vessel.
H2 (g) + Br2 (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are __________ mol of Br2 present in the reaction vessel.
a. | 0.000 |
b. | 0.440 |
c. | 0.566 |
d. | 0.232 |
e. | 0.324 |
4.
Dinitrogentetraoxide partially decomposes according to the following
equilibrium:
N2O4 (g)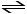 2NO2 (g)
2NO2 (g)
A 1.00-L flask is charged with0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________.
N2O4 (g)
A 1.00-L flask is charged with0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________.
a. | 2.2 ´ 10-4 |
b. | 13 |
c. | 0.22 |
d. | 0.022 |
e. | 0.87 |
5.
In the coal-gasification process, carbon monoxide is converted to carbon dioxide
via the following reaction:
CO (g) + H2O (g)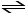 CO2 (g) + H2 (g)
CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is __________.
CO (g) + H2O (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is __________.
a. | 5.47 |
b. | 0.75 |
c. | 1.78 |
d. | 0.56 |
e. | 1.0 |
6.
The equilibrium constant (Kp) for the interconversion of
PCl5 and PCl3 is 0.0121:
PCl5 (g)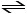 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial pressure of PCl3 is __________ atm.
PCl5 (g)
A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial pressure of PCl3 is __________ atm.
a. | 0.0782 |
b. | 0.0455 |
c. | 0.0908 |
d. | 0.0330 |
e. | 0.123 |
7.
At equilibrium, __________.
a. | all chemical reactions have ceased |
b. | the rates of the forward and reverse reactions are equal |
c. | the rate constants of the forward and reverse reactions are equal |
d. | the value of the equilibrium constant is 1 |
e. | the limiting reagent has been consumed |
8.
Which one of the following is true concerning the Haber process?
a. | It is a process used for shifting equilibrium positions to the right for more economical chemical synthesis of a variety of substances. |
b. | It is a process used for the synthesis of ammonia. |
c. | It is another way of stating LeChatelier's principle. |
d. | It is an industrial synthesis of sodium chloride that was discovered by Karl Haber. |
e. | It is a process for the synthesis of elemental chlorine. |
9.
Which one of the following will change the value of an equilibrium
constant?
a. | changing temperature |
b. | adding other substances that do not react with any of the species involved in the equilibrium |
c. | varying the initial concentrations of reactants |
d. | varying the initial concentrations of products |
e. | changing the volume of the reaction vessel |
10.
Which of the following expressions is the correct equilibrium-constant
expression for the equilibrium between dinitrogen tetroxide and nitrogen dioxide?
N2O4 (g)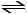 2NO2 (g)
2NO2 (g)
N2O4 (g)
a. | |
b. | |
c. | |
d. | [NO2][N2O4] |
e. | [NO2]2[N2O4] |
11.
Given the following reaction at equilibrium, if Kc = 6.44 x 105
at 230.0°C, Kp = __________.
2NO (g) + O2 (g)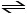 2NO2 (g)
2NO2 (g)
2NO (g) + O2 (g)
a. | 3.67 x 10-2 |
b. | 1.56 x 104 |
c. | 6.44 x 105 |
d. | 2.66 x 106 |
e. | 2.67 x 107 |
12.
Given the following reaction at equilibrium at 450.0°C:
CaCO3 (s)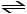 CaO (s) + CO2 (g)
CaO (s) + CO2 (g)
If pCO2 = 0.0160 atm, Kc = __________.
CaCO3 (s)
If pCO2 = 0.0160 atm, Kc = __________.
a. | 0.0160 |
b. | 0.0821 |
c. | 7.23 |
d. | 2.70 x 10-4 |
e. | 723 |
13.
Which of the following expressions is the correct equilibrium-constant
expression for the reaction below?
(NH4)2Se (s)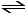 2NH3 (g) + H2Se (g)
2NH3 (g) + H2Se (g)
(NH4)2Se (s)
a. | [NH3][H2Se] / [(NH4)2Se] |
b. | [(NH4)2Se] / [NH3]2[H2Se] |
c. | 1 / [(NH4)2Se] |
d. | [NH3]2[H2Se] |
e. | [NH3]2[H2Se] / [(NH4)2Se] |
14.
Which of the following expressions is the correct equilibrium-constant
expression for the reaction below?
HF (aq) + H2O (l)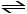 H3O+ (aq) + F- (aq)
H3O+ (aq) + F- (aq)
HF (aq) + H2O (l)
a. | [HF][H2O] / {H3O+][F-] |
b. | 1 / [HF] |
c. | [H3O+][F-] / [HF][H2O] |
d. | [H3O+][F-] / [HF] |
e. | [F-] / [HF] |
15.
The equilibrium constant for the gas phase reaction
N2 (g) + 3H2 (g)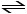 2NH3 (g)
2NH3 (g)
is Keq = 4.34 ´ 10-3 at 300°C. At equilibrium, __________.
N2 (g) + 3H2 (g)
is Keq = 4.34 ´ 10-3 at 300°C. At equilibrium, __________.
a. | products predominate |
b. | reactants predominate |
c. | roughly equal amounts of products and reactants are present |
d. | only products are present |
e. | only reactants are present |
16.
The equilibrium constant for reaction 1 is K. The equilibrium constant for
reaction 2 is __________.
(1) SO2 (g) + (1/2) O2 (g)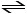 SO3 (g)
SO3 (g)
(2) 2SO3 (g)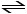 2SO2 (g) + O2
(g)
2SO2 (g) + O2
(g)
(1) SO2 (g) + (1/2) O2 (g)
(2) 2SO3 (g)
a. | K2 |
b. | 2K |
c. | 1/2K |
d. | 1/K2 |
e. | -K2 |
17.
The equilibrium expression for Kp for the reaction below is
__________.
2O3 (g)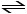 3O2
(g)
3O2
(g)
2O3 (g)
a. | |
b. | |
c. | |
d. | |
e. |
18.
The Keq for the equilibrium below is 0.112 at 700.0°C.
SO2 (g) +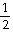 O2 (g)
O2 (g) 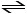 SO3
(g)
SO3
(g)
What is the value of Keq at this temperature for the following reaction?
SO3 (g)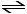 SO2 (g) +
SO2 (g) + 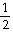 O2 (g)
O2 (g)
SO2 (g) +
What is the value of Keq at this temperature for the following reaction?
SO3 (g)
a. | 0.224 |
b. | 0.0125 |
c. | 0.112 |
d. | 8.93 |
e. | -0.112 |
19.
The expression for Kp for the reaction below is __________.
4CuO (s) + CH4 (g)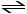 CO2 (g)
+ 4Cu (s) + 2H2O (g)
CO2 (g)
+ 4Cu (s) + 2H2O (g)
4CuO (s) + CH4 (g)
a. | |
b. | |
c. | |
d. | |
e. |
20.
At 400 K, the equilibrium constant for the reaction
Br2 (g) + Cl2 (g)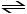 2BrCl (g)
2BrCl (g)
is Kp = 7.0. A closed vessel at 400 K is charged with 1.00 atm of Br2 (g), 1.00 atm of Cl2 (g), and 2.00 atm of BrCl (g). Use Q to determine which of the statements below is true.
Br2 (g) + Cl2 (g)
is Kp = 7.0. A closed vessel at 400 K is charged with 1.00 atm of Br2 (g), 1.00 atm of Cl2 (g), and 2.00 atm of BrCl (g). Use Q to determine which of the statements below is true.
a. | The equilibrium partial pressures of Br2, Cl2, and BrCl will be the same as the initial values. |
b. | The equilibrium partial pressure of Br2 will be greater than 1.00 atm. |
c. | At equilibrium, the total pressure in the vessel will be less than the initial total pressure. |
d. | The equilibrium partial pressure of BrCl (g) will be greater than 2.00 atm. |
e. | The reaction will go to completion since there are equal amounts of Br2 and Cl2. |
21.
Nitrosyl bromide decomposes according to the following equation.
2NOBr (g)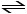 2NO (g) + Br2 (g)
2NO (g) + Br2 (g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or Br2. At equilibrium the flask contained 0.46 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
2NOBr (g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or Br2. At equilibrium the flask contained 0.46 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
a. | 0.18, 0.18 |
b. | 0.46, 0.23 |
c. | 0.18, 0.090 |
d. | 0.18, 0.360 |
e. | 0.46, 0.46 |
22.
In which of the following reactions would increasing pressure at constant
temperature not change the concentrations of reactants and products, based on Le
Chätelier's principle?
a. | N2 (g) + 3H2 (g) |
b. | N2O4 (g) |
c. | N2 (g) + 2O2
(g) |
d. | 2N2
(g) + O2 (g) |
e. | N2 (g) + O2 (g) |
23.
Consider the following reaction at equilibrium:
2NH3 (g)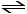 N2 (g) + 3H2
(g) DH° = +92.4 kJ
N2 (g) + 3H2
(g) DH° = +92.4 kJ
Le Chätelier's principle predicts that adding N2 (g) to the system at equilibrium will result in __________.
2NH3 (g)
Le Chätelier's principle predicts that adding N2 (g) to the system at equilibrium will result in __________.
a. | a decrease in the concentration of NH3 (g) |
b. | a decrease in the concentration of H2 (g) |
c. | an increase in the value of the equilibrium constant |
d. | a lower partial pressure of N2 |
e. | removal of all of the H2 (g) |
24.
Consider the following reaction at equilibrium:
2CO2 (g)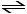 2CO (g) + O2 (g) DH° = -514 kJ
2CO (g) + O2 (g) DH° = -514 kJ
Le Chätelier's principle predicts that adding O2 (g) to the reaction container will __________.
2CO2 (g)
Le Chätelier's principle predicts that adding O2 (g) to the reaction container will __________.
a. | increase the partial pressure of CO (g) at equilibrium |
b. | decrease the partial pressure of CO2 (g) at equilibrium |
c. | increase the value of the equilibrium constant |
d. | increase the partial pressure of CO2 (g) at equilibrium |
e. | decrease the value of the equilibrium constant |
25.
Consider the following reaction at equilibrium:
C (s) + H2O (g)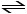 CO (g) + H2 (g)
CO (g) + H2 (g)
Which of the following conditions will increase the partial pressure of CO?
C (s) + H2O (g)
Which of the following conditions will increase the partial pressure of CO?
a. | decreasing the partial pressure of H2O (g) |
b. | removing H2O (g) from the system |
c. | decreasing the volume of the reaction vessel |
d. | decreasing the pressure in the reaction vessel |
e. | increasing the amount of carbon in the system |