Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
In balancing the nuclear reaction 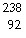 U ® 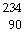 E + 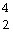 He, the
identity of element E is __________.
|
|
|
2.
|
This reaction is an example of __________. 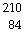 Po ® 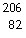 Pb + _____ a. | alpha decay | b. | beta emission | c. | gamma
emission | d. | positron emission | e. | electron
capture |
|
|
|
3.
|
The missing product from this reaction is __________. 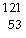 I
® 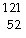 Te + _____
|
|
|
4.
|
What is the mass number of a neutron?
|
|
|
5.
|
What order process is radioactive decay?
a. | zeroth | b. | first | c. | second | d. | third | e. | fourth |
|
|
|
6.
|
131I has a half-life of 8.04 days. Assuming you start with a 1.53 mg
sample of 131I, how many mg will remain after 13.0 days?
a. | 0.835 | b. | 0.268 | c. | 0.422 | d. | 0.440 | e. | 0.499 |
|
|
|
7.
|
The decay of a radionuclide with a half-life of 4.3 ´ 105 years has a rate constant (in yr-1) equal to
__________.
a. | 6.2 ´ 105 | b. | 1.6 ´ 10-6 | c. | 2.3 ´
10-6 | d. | 2.8 ´ 103 | e. | 5.9 ´ 10-8 |
|
|
|
8.
|
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What
is the mass defect (in amu) of a 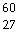 Co nucleus? (The mass of a cobalt-60 nucleus
is 59.9338 amu.) a. | 27.7830 | b. | 0.5489 | c. | 0.5405 | d. | 0.0662 | e. | 0.4827 |
|
|
|
9.
|
Atoms with the same atomic number and different mass numbers
a. | do not exist. | b. | are isomers. | c. | are
isotopes. | d. | are allotropes | e. | are resonance
structures. |
|