Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Based on molecular mass and dipole moment of the five compounds in the table
below, which should have the highest boiling point? 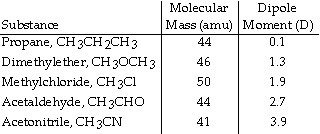 a. | CH3CH2CH3 | b. | CH3OCH3 | c. | CH3Cl | d. | CH3CHO | e. | CH3CN
|
|
|
|
2.
|
Of the following substances, only __________ has London dispersion forces as its
only intermolecular force.
a. | CH3OH | b. | NH3 | c. | H2S | d. | CH4 | e. | HCl |
|
|
|
3.
|
Of the following substances, __________ has the highest boiling point.
|
|
|
4.
|
The substance with the largest heat of vaporization is __________.
|
|
|
5.
|
Of the following, __________ is an exothermic process.
a. | melting | b. | subliming | c. | freezing | d. | boiling | e. | All of the above are
exothermic. |
|
|
|
6.
|
The heat of fusion of water is 6.01 kJ/mol. The heat capacity of liquid water is
75.3 J/mol • K. The conversion of 50.0 g of ice at 0.00°C to liquid water at 22.0°C
requires __________ kJ of heat.
a. | 3.8 ´ 102 | b. | 21.3 | c. | 17.2 | d. | 0.469 | e. | Insufficient data are
given. |
|
|
|
|
|
|
7.
|
The heating curve shown was generated by measuring the heat flow and temperature
for a solid as it was heated. The slope of the __________ segment corresponds to the heat capacity of
the liquid of the substance.
|
|
|
|
|
|
8.
|
On the phase diagram shown above, segment __________ corresponds to the
conditions of temperature and pressure under which the solid and the gas of the substance are in
equilibrium.
|
|
|
9.
|
On the phase diagram shown above, the coordinates of point __________ correspond
to the critical temperature and pressure.
|
|
|
10.
|
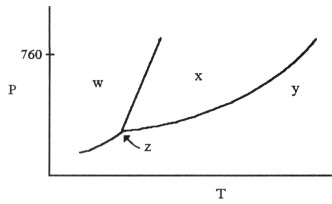
The phase diagram of a substance is
given above. The region that corresponds to the solid phase is __________.
|
|
|
11.
|
Gallium crystallizes in a primitive cubic unit cell. The length of the unit cell
edge is 3.70Å. The radius of a Ga atom is __________ Å.
a. | 7.40 | b. | 3.70 | c. | 1.85 | d. | 0.930 | e. | Insufficient data is
given. |
|
|
|
12.
|
Potassium metal crystallizes in a body-centered cubic structure with a unit cell
edge length of 5.31 Å. The radius of a potassium atom is __________ Å.
a. | 1.33 | b. | 1.88 | c. | 2.30 | d. | 2.66 | e. | 5.31 |
|
|
|
13.
|
In liquids, the attractive intermolecular forces are __________.
a. | very weak compared with kinetic energies of the molecules | b. | strong enough to
hold molecules relatively close together | c. | strong enough to keep the molecules confined to
vibrating about their fixed lattice points | d. | not strong enough to keep molecules from moving
past each other | e. | strong enough to hold molecules relatively close together but not strong
enough to keep molecules from moving past each other |
|
|
|
14.
|
When NaCl dissolves in water, aqueous Na+ and Cl- ions
result. The force of attraction that exists between Na+ and H2O is called a(n)
__________ interaction.
a. | dipole-dipole | b. | ion-ion | c. | hydrogen
bonding | d. | ion-dipole | e. | London dispersion
force |
|
|
|
15.
|
Hydrogen bonding is a special case of __________.
a. | London-dispersion forces | b. | ion-dipole attraction | c. | dipole-dipole
attractions | d. | ion-ion interactions | e. | none of the
above |
|
|
|
16.
|
Which one of the following substances will have hydrogen bonding as one of its
intermolecular forces?
|