Chemical Kinetics (Chapter 14 Brown, Lemay and others 11th ed.)
Quiz
- Which of the following is the primary reason for the increase in the rate of a reaction with an increase in temperature?
- The fraction of high energy molecular collisions increases
- Reactant molecules collide more frequently at higher temperatures
- The pressure exerted by the reactant molecules increases
- The vapor pressure of the reactant molecules increases
- The Activation Energy decreases with an increase in temperature
- Which statement below is true considering the following reaction:
H2(g) + Cl2(g) ==> 2HCl(g)- ΔG is large and positive therefore reaction rate is very fast
- ΔG is large and negative therefore reaction rate is very fast
- ΔG = 0 indicating the reaction rate is not significant
- The ΔG for this reaction is not related to reaction rate
- The "U+0394"G for this reaction is large and positive indicating that the reaction is spontaneous
- If the half-life of a decomposition reaction decreases as the amount of reactant initially present increases, which of the following terms could describe the reaction?
- Zero Order
- First Order
- Second Order
- Exothermic
- Spontaneous
- For which reaction orders is half-life always constant?
- Zero order
- 1st order
- 2nd order
- Both choices B and C
- Choices A, B, and C
- The rate constant _____________?
- always shows an exponential increase with absolute temp (K).
- increases with decreasing concentration.
- usually increases with pressure (for gasses).
- is the same for a given reaction at a specified temperature.
- never changes irrespective of temperature (it is a constant).
- Radioactive nuclear decay is a first order process. An initial sample of 80.0 g of a radioactive isotope is found to decay to 20.0 g after 12 days. When was there 40.0 g of the isotope present?
- After 2 days
- After 4 days
- After 6 days
- After 8 days
- After 10 days
- If both ΔH and Ea for the forward direction are known, the reverse reaction would have and Ea value of _____________?
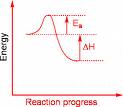
- (-ΔH + Ea)
- (ΔH + Ea)
- ( Ea)
- (- Ea)
- None of these choices describe Ea for the reverse reaction
- Consider the irreversible reaction of Nitrogen Monoxide with Bromine, which is given by the following overall balanced equation:
2 NO(g) + Br2(g) ==> 2 NOBr(g)
The reaction is thought to proceed by the following two-step mechanism:
NO(g) + Br2(g) ==> NOBr(g) + Br(g) (Slow)
NO(g) + Br(g) ==> NOBr(g) (Fast)
Which substance is considered an intermediate of the reaction?- NO(g)
- Br2(g)
- NOBr(g)
- NOBr2(g)
- Br(g)
- What is the expected rate law for the reaction shown in Question 8?
- Rxn Rate = k [NO] [Br2]
- Rxn Rate = k [NO]
- Rxn Rate = k [Br2]
- Rxn Rate = k . [NO]^2
- Rxn Rate = k [NO]^2 [Br2]
- For two 1st order reactions:
A ===> B t(1/2) = 30.0 min
W ===> X t(1/2) = 60.0 min
Which of the following statements is true- doubling A will have 1/2 the effect on half-life that doubling the concentration of B would have
- a certain mass of A will react twice as fast as the same mass of W
- a certain mass of W will react twice as fast as the same mass of A
- the rate constant (k) is lower for A ===> B than for W ===> X
- 4 moles of A will react more quickly than 4 moles of W